Home » Keywords: » defibrillators
Items Tagged with 'defibrillators'
ARTICLES
AEDs 101: Covering the basics
National Institutes of Health answers to frequently asked questions
October 2, 2014
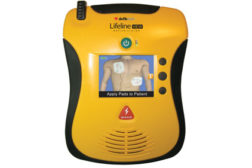
Cleared for sale in U.S., Defibtech Lifeline PRO and ECG AEDs feature revolutionary color LCD video display and 3-lead ECG monitoring capability
Entire family of Defibtech AEDs now available to U.S. customers, providing outstanding lifesaving technology for virtually any need, budget or environment
January 23, 2013
Ready for use
AEDs require championing, device tracking, training & drills
February 2, 2012
Become a Leader in Safety Culture
Build your knowledge with ISHN, covering key safety, health and industrial hygiene news, products, and trends.
JOIN TODAYCopyright ©2025. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing